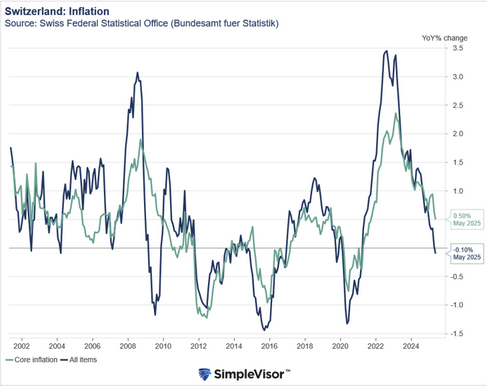
An Indian court has cleared the way for low-cost alternatives to Roche’s drug Evrysdi for spinal muscular atrophy. What’s behind the decision and what could it mean for rare disease patients everywhere? On October 9 the Delhi High Court refused to issue Roche an injunction to prevent generic manufacturers from making and selling a low-cost version of one of the company’s top selling drugs. The drug risdiplam, sold by the Swiss pharma giant as Evrysdi, was first approved in the US in 2020 for the treatment of spinal muscular atrophy – a genetic disease that leads to muscle loss and wasting. Babies with the most serious form of the disease often don’t live past their second birthday. What does the Delhi court’s ruling mean for patients in India and beyond? Alpana Sharma, the founder of the patient advocacy group Cure SMA Foundation India, told Swissinfo via email that the court’s “move has the potential to significantly improve access to affordable treatment options for SMA patients ...
Full story here
Are you the author?
Previous post
See more for
Next post
Tags: Featured,newsletter
Home › 3) Swiss Markets and News › 3.) Swissinfo Business and Economy › Patent battle in India tests Roche’s grip on rare disease market
Previous post
Next post
Patent battle in India tests Roche’s grip on rare disease market
Published on October 16, 2025
Permanent link to this article: https://snbchf.com/2025/10/patent-india-tests-roche-disease-market/
Receive a Daily Mail from this Blog
Live Currency Cross Rates
 On Swiss National Bank
On Swiss National Bank
-
SNB Sight Deposits: increased by 5.2 billion francs compared to the previous week
6 days ago -
SNB’s Chairman Schlegel: A few months of negative inflation wouldn’t be a problem
2026-01-21 -
2025-07-31 – Interim results of the Swiss National Bank as at 30 June 2025
2025-07-31 -
SNB Brings Back Zero Percent Interest Rates
2025-06-26 -
Hold-up sur l’eau potable (2/2) : la supercherie de « l’hydrogène vert ». Par Vincent Held
2025-06-24
 Main SNB Background Info
Main SNB Background Info
-
SNB Sight Deposits: increased by 5.2 billion francs compared to the previous week
6 days ago -
The Secret History Of The Banking Crisis
2017-08-14 -
SNB Balance Sheet Now Over 100 percent GDP
2016-08-29 -
The relationship between CHF and gold
2016-07-23 -
CHF Price Movements: Correlations between CHF and the German Economy
2016-07-22
Featured and recent
-
Entrepreneurship Beyond Politics: Mises Circle in Oklahoma City
-
 What is America’s oldest constitutional debate? | The Economist
What is America’s oldest constitutional debate? | The Economist -
 Linnemann enthüllt SCHOCK-Wahrheit auf Parteitag: “Klingbeil ist SPD Kanzler”!
Linnemann enthüllt SCHOCK-Wahrheit auf Parteitag: “Klingbeil ist SPD Kanzler”! -
 Switzerland, the country of four seas
Switzerland, the country of four seas -
 The Business Cycle Narrative & War With Iran
The Business Cycle Narrative & War With Iran -
 Leben wir in einer Dystopie? #thorstenwittmann #finanzstrategien #finanzen
Leben wir in einer Dystopie? #thorstenwittmann #finanzstrategien #finanzen -
 Cash für Medaillen
Cash für Medaillen -
 Big Banks Get Bullish on Gold, But Some Politicians Get Grabby
Big Banks Get Bullish on Gold, But Some Politicians Get Grabby -
 Achtung: Elon Musk zerstört gerade Wikipedia!
Achtung: Elon Musk zerstört gerade Wikipedia! -
 1776 Patriot Silver Bars Are Selling Out
1776 Patriot Silver Bars Are Selling Out
More from this category
- Entrepreneurship Beyond Politics: Mises Circle in Oklahoma City
21 Feb 2026
 Switzerland, the country of four seas
Switzerland, the country of four seas21 Feb 2026
 The Business Cycle Narrative & War With Iran
The Business Cycle Narrative & War With Iran21 Feb 2026
 Bezos AI firm ‘Project Prometheus’ opening Zurich office
Bezos AI firm ‘Project Prometheus’ opening Zurich office20 Feb 2026
 Salvage begins of Swiss train derailed by avalanche
Salvage begins of Swiss train derailed by avalanche20 Feb 2026
 Tariff Decision Day?
Tariff Decision Day?20 Feb 2026
 4 Percent Inflation: The Case For And Against
4 Percent Inflation: The Case For And Against20 Feb 2026
 Money: The 10 Immutable Laws Of Building Wealth
Money: The 10 Immutable Laws Of Building Wealth20 Feb 2026
 Could direct democracy trip up Swiss trade deals?
Could direct democracy trip up Swiss trade deals?20 Feb 2026
- U.S. Actions Toward Cuba Are Criminal
19 Feb 2026
- Trump Close To a Major Attack on Iran That Will Be Bigger Than 12-Day War
19 Feb 2026
 Links between universities and tobacco industry a threat to Swiss research, NGO warns
Links between universities and tobacco industry a threat to Swiss research, NGO warns19 Feb 2026
 Switzerland mulls discounts on top-selling drugs to cut health costs
Switzerland mulls discounts on top-selling drugs to cut health costs19 Feb 2026
 FX Market Awaits North American Leadership
FX Market Awaits North American Leadership19 Feb 2026
- Trade Deficits and Sound Money
19 Feb 2026
- The Senate and the Loss of “Mixed Government”
19 Feb 2026
- US Actions Toward Cuba Are Criminal
19 Feb 2026
 A No Landing Outcome Is Assumed: Should It Be?
A No Landing Outcome Is Assumed: Should It Be?19 Feb 2026
- French President Macron says free speech is “pure bullsh*t”
19 Feb 2026
 Swiss government announces measures for future food security
Swiss government announces measures for future food security19 Feb 2026