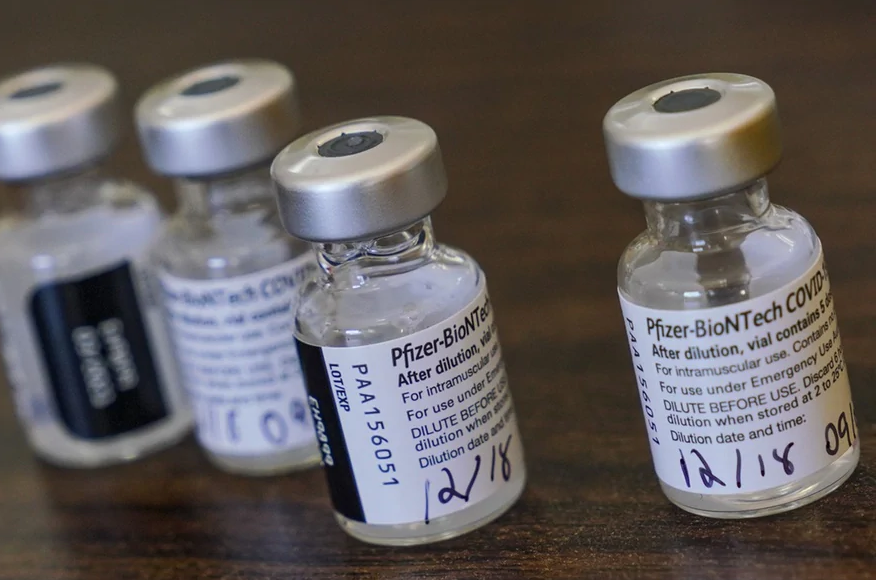
The Covid-19 vaccine from Pfizer/BioNTech is one of three pre-ordered by the Swiss health authorities. Switzerland expects to start vaccinating the most vulnerable people this month already, and nationwide from January 4. Tampa Bay Times
Swiss health regulator Swissmedic has approved the coronavirus vaccine from Pfizer/BioNTech. According to the Swiss authorities, the level of protection is over 90% a week after the second dose.
Two months after receiving the application, Swissmedic, the Swiss Agency for Therapeutic Products, has given the coronavirus vaccine Comirnaty® (BNT162b2) the green light. Based on the available data, the agency found a comparably high level of efficacy in all age groups that were tested.
“The safety of patients is an essential prerequisite, especially where the authorisation of vaccines is concerned,” said Swissmedic Director Raimund Bruhin in a media release on Saturday. “Thanks to the rolling procedure and our flexibly organised teams, we nevertheless managed to reach a decision quickly – while also fully satisfying the three most important requirements of safety, efficacy and quality.”
Anyone aged 16 and up can be vaccinated against the novel coronavirus (SARS-CoV-2), subject to compliance with the federal government’s official vaccination recommendations. For optimum protection, Swissmedic suggests two intramuscular injections of the vaccine, spaced at least 21 days apart.
The authorisation application for Comirnaty®, an mRNA vaccine, was submitted in mid-October and reviewed on an ongoing basis (“rolling submission”). The vaccine has already been approved in Britain, Canada, the United States and other countries.
In addition to Pfizer/BioNTech, Switzerland has signed agreements with vaccine manufacturers Moderna and AstraZeneca. The Alpine country now has 15.8 million doses of vaccines on order from the three makers, pending approval from the health regulator. Swissmedic is also evaluating a vaccine from Janssen-Cilag.
First vaccinations in December
The federal government has ordered around three million doses of vaccine from Pfizer/BioNTech. The first 100,000 doses of vaccine will arrive in Switzerland in the next few days, announced the Federal Office of Public Health on Saturday.
The Armed Forces Pharmacy will store the vaccine at -70 degrees Celsius before distributing it to the cantons, which can store the vials in refrigerators for a maximum of five days.
Canton Basel City will launch its vaccination campaign on December 28, starting with vulnerable residents over 65. Nationwide vaccination is slated to begin on January 4.
Swissmedic’s vaccine approval came earlier than expected. Though it is good news, it also poses additional challenges for Switzerland’s thinly-stretched healthcare system.
It is up to the cantons to decide how to staff and where to provide vaccination services. Sample locations are medical practices, gymnasiums and military facilities. The estimated two million people in risk groups should be vaccinated first, notes the health office. In addition to senior citizens, that includes people with diabetes, chronic lung disease or high blood pressure.
Next in line are medical workers and relatives of the vulnerable. Swiss health authorities say it will be early spring before other people can be vaccinated.
More than 6,000 people in Switzerland (pop. 8.5 million) have died of Covid-19. Over 400,000 have been infected.
Full story here Are you the author? Previous post See more for Next postTags: Featured,Latest news,newsletter