Authorities in many countries are scrambling for solutions to mounting shortages of medicine. Will any of them work?
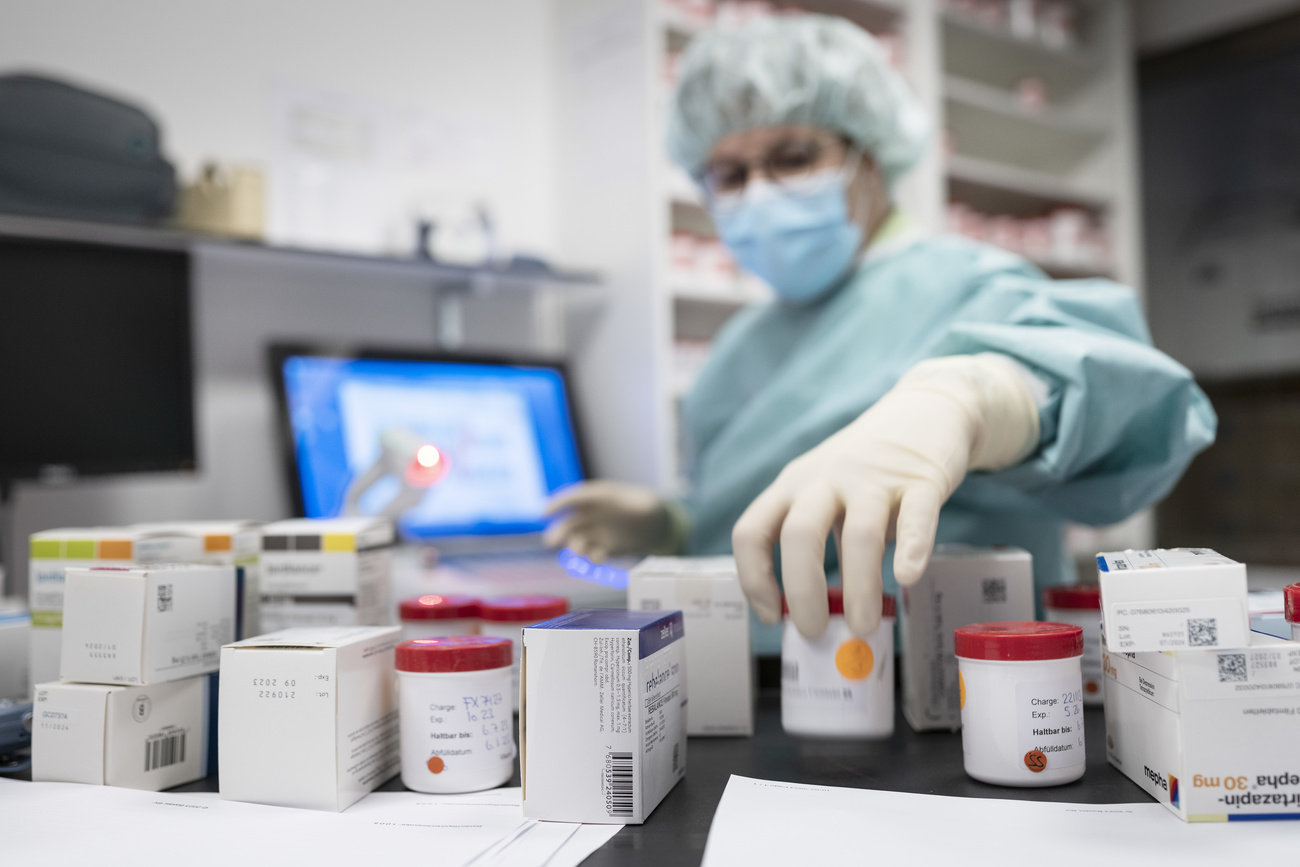
If you haven’t been told by a pharmacist in the last few years that your prescription medicine isn’t available, you should consider yourself lucky. In Switzerland, some 700 medications from antibiotics to painkillers are currently in short supplyExternal link at pharmacies.
The country is far from alone. Countries on all continents are reporting shortages of a range of medicine and their troubling effects on patients. In Mexico, for instance, multiple deaths were reported after shortages of morphine led doctors to draw multiple dosesExternal link from a single vial. In Europe, medicine shortages increased 20-fold from 2000 to 2018 and worsened after the pandemic upended supply chains. Medicine isn’t just missing more often, report pharmacists, but also for longer periods of time.
“We [pharmacists] have been complaining about shortages for years,” said Ilaria Passarani, who heads the Pharmaceutical Group of the European Union, representing more than 400,000 community pharmacists. “The situation has gotten so bad that it now has the attention of policymakers and the media.”
Pharmacists have become much better at troubleshooting – looking for alternatives or rationing supplies. These are only band-aids though, and increasingly costly ones. In Europe, pharmacists set aside 10 hours External linka week just looking for solutions to medicine shortages.
Health authorities globally are now debating possible solutions, including some that address the root causes of shortages such as the globalised medicine supply chain. Switzerland too is witnessing a growing push to address the issue.
A coalition of doctors, pharmacists, and consumer protection agencies in Switzerland gathered the necessary 100,000 signatures to launch a popular initiative on medicine shortagesExternal link last October. The initiative calls for a new article in the Swiss constitution on medicine supply security. It would require the Swiss government to create framework conditions to prevent shortages. This should come to a nationwide vote in the next couple years.
How much hope do the proposed solutions offer? We looked at five options.
1. Creating better “early warning” systems
One of the low hanging fruits for dealing with medicine shortages is getting a better handle on the shortages themselves. This includes monitoring and early warning systems so that pharmacies and doctors can stockpile, forecast, and look for alternatives before it affects patients.
The European Medicines Agency plans to launch the European Shortages Monitoring Platform (ESMP) in February that will centralise and automate alerts across the bloc. Brazil also introduced real-time monitoring softwareExternal link across the country called MonitoraAF in 2022.
Swiss authorities plan to modernise their own monitoring platform for vital medicines to make it more of an early warning system although it hasn’t specified how this system would work.
These measures will help authorities become more aware of shortages as they happen, but they still lack key elements to manage the situation, said Valérie Junod, a professor of pharmaceutical law at the universities of Lausanne and Geneva, who co-wrote a paperExternal link on medicine shortages in 2021.
There’s no central management of supply and demand, at a local and country level let alone at a global level. “Each hospital, each pharmacy is looking for solutions on their own. One hospital might decide to switch to another drug but what happens if everyone does?” said Junod. “It’s still a piecemeal approach.”
There’s also very little transparency into why a specific shortage is happening, she added. Drugmakers don’t provide information about production locations for specific medicine and vulnerabilities. This makes it difficult for authorities to identify risks and anticipate shortages and their duration.
2. Allowing substitutes
Another immediate solution under discussion in many countries is giving pharmacists and doctors more flexibility to offer alternative medicine. Pharmacists are restricted by law in some countries from suggesting a smaller package or different dosage forms – for example, a syrup instead of a pill.
Switzerland recently made it easier for pharmacists to substitute medicine and import foreign alternatives in case of serious shortages. There’s also debate about allowing medicine to be used beyond its expiration date, rather than discard it when studies show it’s still safe.
Some countries, including Switzerland, Japan and South Africa, also allow so-called “compounding pharmacies” to produce small doses of some generic medicine when out of stock. Europe is taking this a step further. The Council of Europe has created the European Drug Shortage FormularyExternal link, which will provide guidance for pharmacists on preparing unlicensed medicine (those not approved locally but have the same active substance) during shortages.
This could be particularly useful for generic drugs. However, the situation is more complicated for more expensive, patented drugs such as the weight-loss drug Wegovy.
Debates are raging in the United States about whether pharmaciesExternal link should be allowed to produce so-called “compounded versions” or copies of drugs when manufacturers can’t meet supply. US drug authoritiesExternal link argue this is allowed when not done “regularly or in inordinate amounts”. Pharmaceutical companies have pushed back on moves to open the market to more drugmakers, arguing this infringes on their drug patents.
3. Making the market more attractive
A more long-term solution to shortages requires addressing underlying causes, including that the market for cheap, generics, which make up the majority of out-of-stock medicine, isn’t attractive.
Some drugs are so cheap that companies don’t find them worth producing. This has left few suppliers for many drugs, and a lot of outsourcing of final drugs and active ingredients to cheaper locations.
A studyExternal link published last year found that since 2022 at least 18 countries are providing financial incentives to drugmakers, usually in the form of price increases of some off-patent medicines. This includes Brazil, which exempted some medicines from price regulation for at least a year.
More
More
Why Switzerland is running out of pharmaceuticals
Small markets like Switzerland face more challenges because even higher prices can’t make up for low volumes. The Swiss parliament is talking about making exceptions to the regular three-year timeline to review drug prices, when prices are usually lowered. There are also efforts underway to lower costs by introducing a QR code on packages instead of printed instructions in three languages.
Higher prices aren’t without criticism. Raising prices at a time of mounting healthcare costs is politically sensitive and can place a huge burden on patients.
The other concern is that authorities have little visibility into drug prices and profitability. This makes it difficult to know what price would lead manufacturers to stop producing or supplying a drug.
4. Bringing back production of cheap generics
Another widely discussed solution to shortages is reshoring or boosting local production of older drugs. Up to 80% of active ingredients and about 40% of finished medicines sold in the EU come from China or India. This has left supply chains vulnerable to export restrictions and other disruptions.
Many governments are now trying to find ways to boost local production without raising costs. In summer 2023, the French government announcedExternal link it aims to bring back production of 50 vital medicine, where it depends on imports. Public money to the tune of €160 million ($151 million) will support eight new production projects, including one for the antibiotic amoxycillin (produced by British pharma giant GSK in northwestern France), as well as painkillers and cancer drugs.
Both Russia and KazakhstanExternal link have set a goal to produce 50% of medicine domestically by 2025 and Saudi Arabia aims to produce 80% of pharmaceuticals locally by 2030.
The Austrian government has also provided a €28.8 million grant to Basel-based generic maker Sandoz to support production of the antibiotic amoxicillin in the country. The Brazilian government has also provided financing to state-owned pharmaceutical companies, such as Fiocruz, to produce essential medicines domestically.
Some countries, including Switzerland, are wary of direct subsidies or taking stakes in companies. They prefer to rely on creating an attractive business environment, specifically high skilled workforce and tax benefits. For example, US firm Biogen’s CHF1.5 billion ($1.65 billion) investment in a biologics factory in Switzerland benefited from tax incentives and local investments in sustainable energy sources.
Experts are doubtful that reshoring production would even eliminate shortages though. “Bringing production back to Europe will reduce the complexity of supply chains as well as the risks incurred in long transports,” said Petra Doerr, the director of the European Directorate for the Quality of Medicines & HealthCare (EDQM), told SWI.
But what’s most important is diversification of suppliers, especially of active ingredients that are currently concentrated in the facilities of a small number of suppliers. “If one source fails, then another can meet the demand,” said Doerr.
5. Building secure supply chains for future drugs
Some of the immediate solutions such as better monitoring and stockpiling have started to ease acute shortages in some countries.
The larger question is what to do now to prevent shortages for future drugs. Drugmakers are looking at advanced manufacturing systems to reduce disruptions and lower production costs, as well as using more artificial intelligenceExternal link to improve supply and demand forecasting. Experts also emphasise that global collaboration will be needed
However, this still doesn’t guarantee that, when drugs go off patent, drugmakers won’t pursue the same low-cost supply chain strategies that have led to the shortages.
There are times when authorities can’t solely rely on voluntary efforts by companies, said law professor Junod. Authorities can introduce supply commitments and penalties for not meeting them. In a reportExternal link on shortages published in 2021, she suggested that supply security should be a criteria for authorising a medicine and, in case of violation, that “company sanctions should be set as a percentage of sales, taking into account the seriousness and impact [of shortages] on patients”.
The US and France have already issued fines for companies failing to notify shortages. India’s government has blacklisted pharmaceutical companies from bidding on public procurement contracts for failing to maintain supply or produce substandard medicines. The Swiss government said last AugustExternal link that it is “examining whether remuneration or approval can be linked even more closely to the criterion that the supply of this medicine is guaranteed”.
“Even in a small country, threats and incentives can make companies behave differently,” said Junod.
Edited by Virginie Mangin/ds
More
Tags: Featured,newsletter