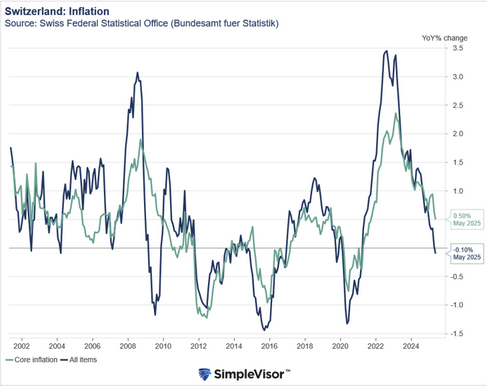
The United States Food and Drug Administration (FDA) published Thursday a list of pharmaceutical companies that include Basel-based Novartis and Roche, all of which are suspected of hindering the development of generic versions of their own medicines. Over 150 complaints were filed.
The FDA slammed the development of techniques designed to complicate or even prevent developers of generic drugs from accessing sufficient quantities of original preparations to be able to do comparative studies essential to any application for approval.
The authority said it has received more than 150 complaints over the alleged misuse of FDA programmes or agreements between pharmaceutical companies and distributors.
The FDA noted that it had not investigated these allegations.
Full story here Are you the author? Previous post See more for Next postTags: Business,newslettersent